Revitalisation Organique
CELLORGANE 4G a incorporé dans ses différentes présentations et formules des biopeptides pour renforcer davantage l'efficacité de ces traitements. Ces nouveaux composants augmenteront leur pouvoir thérapeutique dans le comportement du cycle cellulaire car ils ont une influence sur l'autorégénération des tissus et des organes, stimulent les processus d'oxydation et de réduction, devenant ainsi un véritable neutralisant des substances toxiques dans les cellules. Ainsi, le traitement offre une triple activité dans l'ordre de l'efficacité thérapeutique, visant à améliorer les fonctions des organes et des systèmes en état de dysfonctionnement ou de maladie en raison d'un affaiblissement ou d'autres types de pathologies liées au vieillissement.
Thérapies Biologiques de Vieillissement et de Renouvellement Cellulaire
CELLORGANE 4G est un traitement coadjuvant dans la revitalisation des tissus et des organes. En réalité, il s'agit d'une hypernutrition cellulaire et/ou d'une nutrition cellulaire catalysée qui renforce et stimule les processus naturels de régénération organique du corps, revitalisant ainsi l'organe usé.
Contrairement aux pratiques médicales conventionnelles actuelles par l'administration de médicaments chimiques de synthèse, la thérapie avec CELLORGANE 4G (qui complète souvent la thérapie conventionnelle dans de nombreuses pathologies) constitue véritablement un traitement biologiquement naturel. C'est là, sans aucun doute, le "cœur" de son effet revitalisant.
Les autres composants de ses formules activent notablement les réponses organiques, restaurant et revitalisant non seulement directement l'organe diminué, mais exerçant également une fonction positive dans le reste des organes qui complètent ce système organique. Exerçant des effets symptomatiques à moyen et long terme, son utilisation dans le cadre de traitements prolongés produit donc des résultats remarquables.
Qu'est-ce que Cellorgane 5 de la 4ème génération ?
Il s'agit d'un traitement d'origine biologique de qualité pharmaceutique, coadjuvant dans la revitalisation des tissus et des organes, offrant une hypernutrition cellulaire et/ou une nutrition cellulaire catalysée, renforçant et stimulant les processus naturels de régénération organique, revitalisant ainsi l'organe usé.
Quels sont les composants de Cellorgane 5 de la 4ème génération ?
Il s'agit d'une combinaison d'extraits cellulaires opothérapeutiques, de biopeptides, d'enzymes et de précurseurs hormonaux qui génèrent d'importants stimuli dans les cellules pour un fonctionnement optimal de divers organes et systèmes. Sa formule est un brevet exclusif à l'échelle mondiale et ne contient pas de produits chimiques, de stéroïdes ni de substituts hormonaux d'origine synthétique.
Comment fonctionne Cellorgane 5 ?
Il fonctionne selon le principe de la "spécificité de l'action". Cela signifie que les extraits cellulaires d'organes spécifiques des formules associés à de petites chaînes d'acides aminés des biopeptides ont la propriété de cibler l'organe ou le tissu malade du patient (organotropisme) agissant sur eux (organospecificité) en induisant la revitalisation avec un fort potentiel régénératif, notamment chez ceux qui entrent en dysfonctionnement ou sont affectés par divers facteurs tels que l'âge, l'hérédité, les processus inflammatoires et pathologiques.
Quels sont les effets secondaires et les réactions de Cellorgane 5 ?
Il n'a pas d'effets secondaires, car sa toxicité est complètement nulle, il ne provoque aucune réaction indésirable et peut être pris en toute sécurité en association avec d'autres traitements, car il n'interagit pas avec d'autres médicaments.
Cellorgane 5 4G est-il disponible en pharmacie ?
Comme il s'agit d'un produit d'origine biologique et non pharmaceutique, il n'est pas en vente dans les pharmacies traditionnelles, seulement dans certaines pharmacies spécialisées.
Biopharmaxie et
Schweizer Klinik Biocell
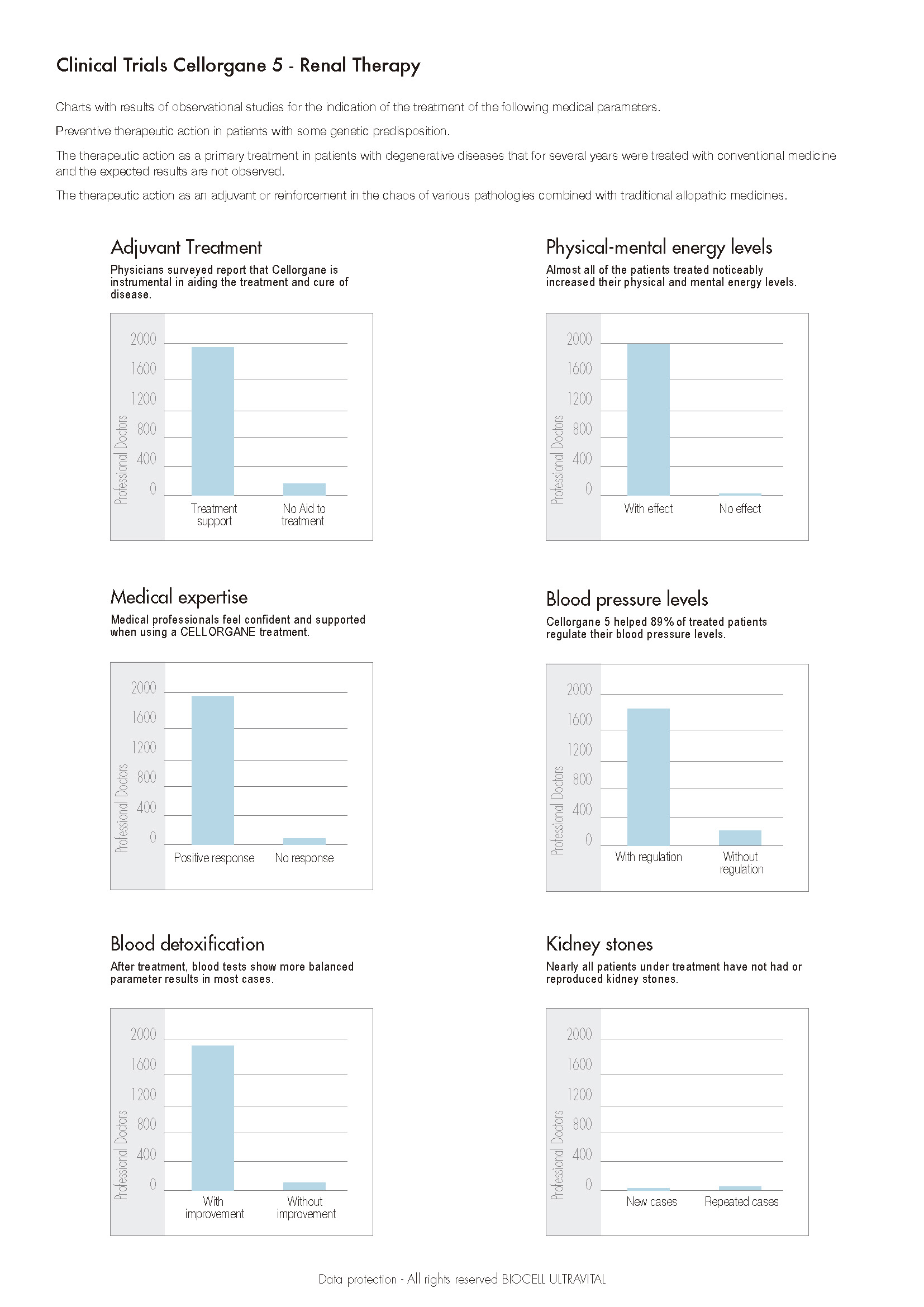
Quels sont les résultats des expériences cliniques avec Cellorgane 5 ?
Cellorgane 5
Indications:
- Insuffisance rénale.
- Anémie.
- Diabète sucré.
- Renforce toutes les fonctions rénales.
- Augmente et normalise la détoxification du sang.
- Maintient l'équilibre général des électrolytes dans le corps.
- Régule la pression sanguine et le volume plasmatique.
- Favorise la décalcification des calculs rénaux et prévient leur formation.
Composition:
Blister de 30 comprimés de 650 mg. / Contenant de 60 comprimés de 650 mg.
Chaque comprimé de 650 mg contient:
Extraits cellulaires opothérapeutiques: Rein, Glande thymus, Placenta.
Extraits de peptides de: Rein, Glande thymus, Placenta.
Complexes enzymatiques: Superoxyde dismutase, Glutathion peroxydase, Glutathion réductase, Glutathion transférase, Adénosine triphosphate (ATP).
Autres ingrédients: stabilisateurs et excipients.
Mécanisme d'action de la 4e génération cellulaire:
Le mécanisme d'action des peptides est similaire à celui des médicaments, car les trois derniers résidus d'acides aminés adjacents à la région C-terminale des peptides présentant une activité thérapeutique sont fortement liés au site actif, où ils ont une plus grande spécificité d'inhibition. Les biopeptides sont protégés dans les comprimés et les capsules par une couche entérique pour éviter leur dégradation dans le tractus gastro-intestinal et favoriser leur meilleure absorption dans les parois intestinales, bénéficiant ainsi du pourcentage d'absorption dans le sang. Pour ce faire, nous avons incorporé des catalyseurs dans chaque formule et trois acides aminés, l'arginine, la glycine et l'acide aspartique, pour augmenter la réaction chimique du corps pour le couplage et l'absorption ultérieure. En incorporant cette séquence dans les différentes chaînes de peptides à différentes positions du type de formule, cela permet de nouvelles variantes à la fois dans la chaîne latérale chargée négativement de l'acide aspartique et dans l'arginine, avec une charge positive. Cette formule et ces méthodes obéissent à un brevet exclusif des produits de 4e génération de Biocell Ultravital pour améliorer leur absorption lorsqu'ils sont administrés par voie orale pour se lier à la molécule cible, et leur effet thérapeutique est restauré lorsqu'ils atteignent leur destination.
Pharmacocinétique et pharmacodynamique:
Les composants des différentes formules atteignent les cellules, soit par voie sanguine directe ou indirecte dans le cas des produits par voie orale, et sont incorporés par elles, par le biais des divers moyens de transport cellulaire, en fonction de la taille des molécules des éléments en question. Dans le cas de grosses molécules, leur incorporation dans les cellules se fera par endocytose médiée par des récepteurs, suivant la chaîne: vésicule, endosome et lysosome. Dans le cas de molécules plus petites, leur incorporation se fera par simple diffusion ou diffusion facilitée par des protéines, selon le cas.
Les extraits cellulaires qui sont entrés dans les cellules par endocytose sont constitués de molécules dont les atomes sont liés par des liaisons chimiques, dans lesquelles l'énergie est retenue. La cellule utilise à la fois la matière et l'énergie, par le biais d'un processus appelé digestion cellulaire, qui décompose les molécules par l'action des enzymes hydrolases contenues dans les lysosomes.
Administration:
Utilisation par voie orale, à consommer avant les repas pour améliorer l'absorption, de préférence avec de l'eau.
Posologie:
En tant que traitement préventif pour les personnes en bonne santé, il est recommandé de prendre un (1) comprimé le matin et un (1) comprimé le soir quotidiennement pendant six mois continus ou intercalés. Pour les personnes ayant une quelconque affection ou maladie indiquée ici, il est recommandé de prendre deux (2) comprimés le matin et deux (2) comprimés le soir quotidiennement pendant douze (12) mois en complément des médicaments indiqués par le médecin traitant. Il peut être pris indéfiniment car ses composants ne créent pas de dépôt.
Effets secondaires:
En raison de sa teneur en peptides et en opothérapie, il pourrait provoquer dans certains cas un léger mal de tête, qui disparaît après quelques minutes, les nausées sont très occasionnelles et disparaissent en quelques heures.
Extraction et obtention de biopeptides et d'extraits cellulaires:
Il convient de noter que les formules contiennent des composants biologiques d'origine animale en combinaison avec d'autres composés qui renforcent l'action thérapeutique et qui sont extraits notamment à un stade embryonnaire et traités au cours du deuxième mois de gestation. Ces composants font partie des formules de certains de nos produits et sont obtenus après un long processus biochimique. Ils sont certifiés exempts de prions, de pyrogènes, de bactéries, de nanobactéries, de champignons, de virus et, après de nombreux contrôles, les risques de réaction immunitaire chez l'homme sont éliminés à 100 %.
De nombreuses années d'expérience clinique ont montré que les porcs et les moutons sont les meilleurs donneurs, car ils sont forts et immunologiquement résistants. La preuve en est que même au 21e siècle, la plupart des greffes de valves cardiaques pour les humains proviennent des porcs, en plus de l'insuline et de nombreux produits dérivés à des fins thérapeutiques, qui proviennent de ces animaux. Ces méthodes sont continuellement surveillées pour déterminer si elles répondent aux normes les plus strictes requises par la biosécurité à des fins thérapeutiques. Tous les composants et autres ingrédients actifs des formules sont également approuvés par la Food and Drug Administration (FDA) et sont fabriqués aux États-Unis par BIOCELL ULTRAVITAL USA sous licence suisse de Biocell Ultravital.
Contre-indications:
Il peut être combiné en toute sécurité avec d'autres médicaments. Dans les doses recommandées, aucune réaction indésirable n'a été observée dans aucun cas. Les personnes atteintes de diabète doivent d'abord être contrôlées.
Présentation et emballage:
Il contient une boîte avec deux (2) plaquettes ou quatre (4) plaquettes de 15 comprimés de 650 mg chacune, et sa fabrication se fait dans une atmosphère stérile conformément aux réglementations internationales.
Conservation:
À conserver dans un endroit sec et frais, à une température ambiante entre 5°C et 40°C. Chaque capsule a une durée de conservation de cinq (5) ans à compter de la date de fabrication.
Contrôle du produit fini:
Deux (2) laboratoires indépendants réalisent divers tests et contrôles fongiques, biologiques, bactériologiques de type 5, analyses immunocellulaires multicentriques, anti-brucellose, contrôles virologiques, tests anti-prion multiples, bactérioses, cyclospores, mycobactéries, salmonellose, conformément à la réglementation actuelle de l'Union européenne concernant les produits biologiques à des fins thérapeutiques pour la consommation humaine.
Attention:
Biocell Ultravital garantit la pureté et la qualité de ses produits et n'est pas responsable des dommages causés à des tiers qui pourraient résulter d'une mauvaise pratique.
How Does it Work?
How the Formulas are Developed?
All our products have been formulated to optimize cell function because if we have healthy cells, we will be healthier every day, and they are based on our 4 pillars of cellular longevity. They work together as a whole to promote energy production and cell growth.
We strive to achieve perfection for each of our therapeutic categories, since they are formulated by carefully selecting each component after confirming its characteristics and clinical trials that positively compromise the efficacy in each formula, this is basically the science on which our products and therapies are based.
Biocell Ultravital products are formulated with ingredients that are 100% natural biological substances, with the highest quality standards, containing cellular extracts of animal origin and peptide hydrolysates extracted from plant extracts and glands of young animals in combination with antioxidants, enzymes and vitamins we managed to optimize the formulas and their therapeutic scope.
All raw material sources are carefully selected from organic plants and organs of pigs and sheep less than one year old and undergo a prion and decontaminated pharmaceutical standardization process that meets high biosecurity standards for reach specific concentrations of high purity and are processed under strict controls to guarantee the high quality and purity of their products.
No chemicals, other harmful or unwanted substances are used in the extraction. Modern technology ensures the recovery of assets in a biologically natural way, without changes. This is very important for the safety of each formula. None of the components in Biocell Ultravital formulas are genetically modified. (NON GMO)
Production and Extraction of Bio Peptides 4th Cellular Generation
The chemical synthesis to produce bioactive peptides of the 4th cell generation of
Biocell Ultravital are obtained using combined methods of enzymatic hydrolysis through proteolytic enzymes and by fermentation using starter cultures. This combination of both methods gives origin to peptides with a safer biological activity due to the fact that in one all the contaminating traces are eliminated and the other facilitates the absorption through the intestinal tract. An additional advantage of enzymatic hydrolysis is the reduction of allergens. The procedure to obtain, isolate and identify biopeptides with specific biological activity, is obtained with the solution of the gland or organ to extract biopeptides, to which papain, pepsin, trypsin are added, the application of different enzymes pursue the formation of a mixture with different ranges of biological activity and incubated for a certain time to obtain the desired degree of hydrolysis.
After this, the hydrolyzate is fractionated by exclusion chromatography and semi-preparative liquid chromatography in reverse phase, selecting the fraction with the highest activity as a result of the first invitro tests, and finally the sequence of the peptides responsible for generating the activity is identified. The desired therapy is obtained by applying mass spectrometry and / or N-terminal sequencing. However, hydrolysates are complex mixtures and can contain up to hundreds of different molecules, therefore, locating bioactive peptides in these procedures is a biochemical work of constant expertise that results in a difficult task and requires a lot of time and dedication. The fractions usually still contain multiple compounds that require additional cycles of fractionation, concentration and evaluation of bioactivity to be able to identify the molecule responsible for the activity in the formulas of each product according to the therapeutic indication.
Consequently, the enzymatic hydrolysis used offers undoubted advantages, such as the absence of substrate degradation processes since the enzymes are selective for a type of bond, pH values between 5 to 10 and the temperatures between 40 to 60ºC, thus maintaining or improving the nutritional value of the protein. The specificity of the enzyme affects the size, quantity, and composition of free peptides and amino acids, as well as their amino acid sequence.
Safety in Therapies
Safety control is a very important factor, especially when it comes to high quality health products, which is why at
Biocell Ultravital in France, which is the main headquarters where all the research and development of the formulas take place, we have monitoring and follow-up mechanisms for each product, this to prevent any error in the next production, that is why we are continuously implementing software for the detection of technical failures of the human team in charge of production. This translates into maximum safety controls that guarantee the complete quality of the production, eliminating 100% possible adverse reactions due to the indication of some of our products that does not correspond to a contraindication already described.
This discipline has become responsible for quality control in the formulation and manufacturing phases thanks to the detection, evaluation, knowledge and analysis for the prevention of adverse reactions and other possible problems related to the products, covering a wide spectrum of continuous analysis so that they do not cause any harm to the patient. We actively participate in decision-making when manufacturing to prevent possible complications in any of the production stages. Thanks to the standardization and development of specific protocols established for each production and subjected to continuous analysis adhering to very specific guidelines we are able to prevent these unwanted complications. We are mandated by compliance with the European Pharmacopoeia in Europe and adhere to current FDA regulations when manufacturing the different products corresponding to Biocell Ultravital Cellular Renovation Therapies.
Research and Development
When
Biocell Ultravital incorporates products with new formulas and they reach the market, at least an average of 5 to 7 years have elapsed, consumed in the different phases of research and development. This journey requires a scientific and economic effort, for this reason we say that out of every 300 new formulas that are beginning to be investigated, only 20 end up becoming treatments available to patients either as an adjunct or preventive of cellular function and the immune system.
Working in the context of formulas based on opotherapeutic extracts or cells of xenogeneic origin, they aim to support the expansion of products for basically strengthening the immune system.
Cellular Renovation Therapy may contribute as reinforcement to the primary treatment to treat the various human diseases that are associated with failures of the immune system. In order to achieve this purpose the continuous efforts of a scientific committee structured by various institutions in the field biology headed by Prof. Dr. Ben L Pfizer is needed. These contributions guide new formulations that improve the components has been allocated to four fundamental guidelines with specific objectives corresponding to the 4th generation cellular products by Biocell Ultravital: Protection, Repair, Revitalization and Regeneration properties. These therapeutic lines are developed with a common research and development strategic plan for each product category.
Manufacturing Plants and Procedures
In Europe, research, production and development is carried out at Biocell Ultravital, France, and we share part of the manufacturing in two other associated plants, mainly for the production of some raw materials, between Germany and the Netherlands.
We also manufacture our products in the USA, BIOCELL ULTRAVITAL USA Laboratories, where we have a modern production factory especially for manufacturing formulas and products for oral presentation.
Since our products formulas require components be extracted from a biological source, in all manufacturing plants we have ensured that they are manufactured in production rooms that have state-of-the-art equipment controlled by software that manages the procedures monitoring each phase of the process. Development with the latest air conditioning, quality and safety systems, to ensure that the final product does not lose its therapeutic potency, for this reason the active ingredients of each formula and the preparation in the initial phase are conditioned in isolated clean rooms and by specialized professionals trained to measure and control the production process from the moment this first manufacturing phase begins and continuing these quality controls through each phase of production until the final product is approved.
Each manufacturing plant, although with different facilities, produces with the same manufacturing procedures, efficiently following the highest quality standards that comply with all the regulations established by the European Pharmacopoeia and the FDA.
Products that Improve Cellular Renovation
Currently there is a growing interest in cell therapy extracted from the patient’s own stem cells, unlike our products,
Cellular renovation therapies (CRT) provide sustained results without complications and represent a great economic saving in comparison. The products in the Biocell Ultravital line of
Cell Renewal Therapies are divided into 5 main groups that include:
Regeneration, Revitalization, Detoxification, De-inflammatory and Hormonal.
CRT have worked with great success in recent years as a preventive treatment, without discarding its effective action as a primary therapeutic. The treatment benefits are notorious for their adjuvant action in allopathic medicine. CRT, through their formulas, exclusively allow us to transform diseased or aging organs into healthy organs with a highly functional capacity. Their components activate a series of stimuli that cells need for a normal cycle of continuous renewal, and they can be incorporated into the body through injections or orally. These various components are assimilated by the body through endocytosis and a good part is introduced into the cells. This is produced by the emission of pseudopods until they completely encompass it to form a vacuole, which then fuses with the lysosomes to degrade the phagocytosed substance, which is known by the name of phagosome. This method is characterized by being the mode of nutrition used by cells through ingestion of foreign matter. In addition, it is one of the greater means of transport that they use to defend themselves against some cells of multicellular organisms.
From this mechanism and many other stimulating biochemical changes, nutrition begins for an effective CELLULAR RENOVATION. CRT brings a fresh genetic information contained in DNA and RNA, stimulating secretion inducing old or sick cells and reprogramming to operate properly, providing the recipient bodies, a large number of biochemical and enzyme substrates containing information needed to revitalize an organ, or a gland. CRT contains various compounds that are primarily responsible for properly nourishing cells, but, moreover, each of the formulas in a CRT contains cells and embryonal tissues that increase positive changes in cell cycle complementing in part dysfunctional lapses. This is how the different components are first incorporated as essential nutrients and as well, how the embryonic tissues reach the organ with low vitality and that need to be dynamized. This process is called CELLULAR RENOVATION and its benefits are perceived after several weeks, as it passes slowly from the cell to the tissue, from the tissue to the organ and from the organ to the system. Some specialists feel that the most important result of using CRT is the revitalization of the body’s immune defense mechanisms. When damage occurs to the cells that make up the different tissues and organs involved in the immune system, either through aging or environmental poisoning, the body becomes defenseless from both external invasion and internal degeneration. The damage caused to the organs of the immune system can be reversed by stimulating the body’s defense mechanisms to boost the health of the weakened organ.
Security System to Prevent Falsification
Biocell Ultravital has developed for the
4th generation a sophisticated security system for its products to prevent the repetition of copies and / or counterfeiting of our products. Moving forward, we will invest a great technological effort in each package to ensure that you will have an original product in your hand.
This security system is applied to new packaging that have a QR Scan code applied to a double security system, a first physical stage in the packaging that will reveal through a security tab if the product has been violated and a second with an implantation of sophisticated software that will allow you to check if the product you purchased is original through our website, or by downloading the new BIOCELL APP for Android and IOS for free.
The falsification of ethical and health products is a problem that is becoming increasingly important, and all the different therapeutic areas may be affected.
These counterfeits seek to pass for authentic products, copying the packaging material, the commercial image, the logo, the information brochures, as well as the shape and colors of the original product.
In addition, they use the same lot number, expiration date and other identifying elements of the authentic product. However, they are manufactured and placed on the market without the authorization of any health authority. These original and generic medicines, as well as vitamins are also falsified and even more so those products that enjoy prestige are the most vulnerable and these falsifications may contain active ingredients in incorrect doses or lower doses than those authorized, or the absence of active ingredients and even include some toxic elements. Many may even be manufactured under dangerous conditions that are not controlled. Thus, they can pose a significant risk to health and are sometimes especially difficult to identify.
In short, they look identical to the original product, but contain “uncontrolled” components that are life threatening.
he trade in counterfeit medicines is controlled by international criminal networks, and no region is exempt. Those countries with less controlled drug distribution systems are more vulnerable to this type of practice; However, the most developed countries are not spared from this evil, especially when patients purchase drugs on the internet or unauthorized websites. Unfortunately, in the past we have been victims of these unethical organizations and have had to report these facts to the authorities since many people especially in Asian countries, as well as Mexico and others in Central and South America are victims of misleading offers of therapies, even by unethical doctors who offer the products at prices lower than the real cost. However, in the end they spend more money due to the need to seek immediate clinical help as demonstrated on several occasions reported by us through our attorneys around the world from patients who were treated clinically for administering fake products.