Clinical studies are applied in the pharmaceutical industry, to specifically determine the action and evolution of the product and its final behavior in the body through pharmacokinetics and pharmacodynamics using a process where up to 4 phases of the drug are commonly involved with the end goal to develop a protocol and measure different interactions.
Biological products such as cell therapies have some characteristics compared to small molecule drugs. These products require novel study designs to address their uniqueness. Close attention to detail must be paid when defining critical endpoints. Special monitoring and reporting should also be considered due to the safety issues associated with these products, especially long-term monitoring, because cell therapies are even more complex and difficult to apply compared to conventional clinical studies as a consequence of the nature of the product. However, we must consider the importance that these therapies have contributed in the last 25 years, especially in revitalizing regenerative medicine, providing significant improvements in the quality of life of patients who have undergone these treatments for various causes and diseases. The mode of action is not always perfect, and the potency tests are still imprecise, hence the main concern when reviewing any clinical trial on allogeneic transplantation in autologous or donor cell therapies and also those of animal origin Xenotransplantation is that they all enjoy 100% biosecurity and with minimal risk of immune rejection.
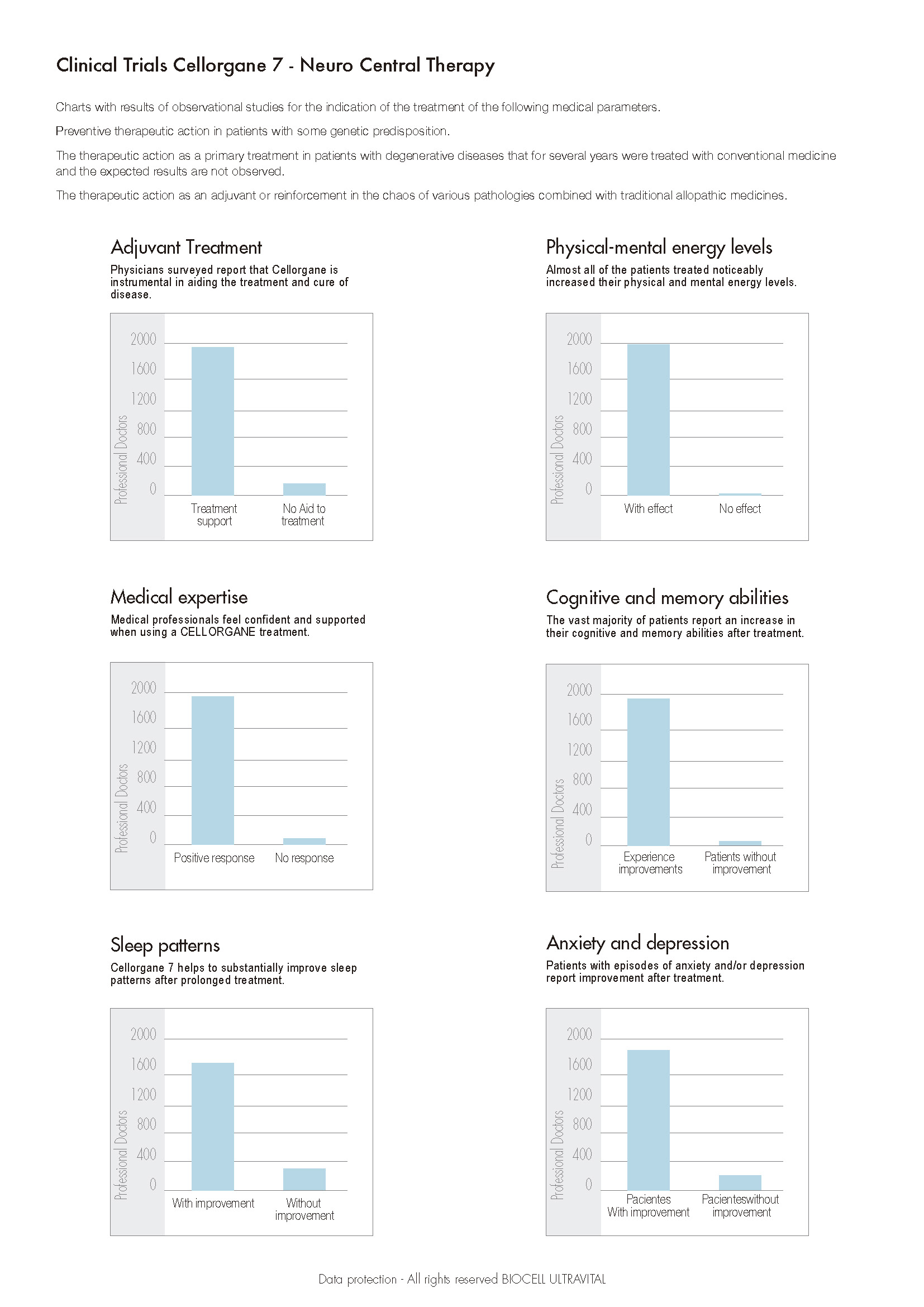
The aforementioned does not relate to Biocell Ultravital products since they use peptides and cell extracts in their formulas that guarantee 100% safety without the possibility of immune rejection in patients. We have accumulated for more than 70 years of varied clinical expertise through specialized doctors mainly in Europe who have used Cellular Renovation Therapies for more than 3 generations to establish safety and efficacy parameters, mainly on patients for which an adequate evaluation is required to determine various aspects. Such as, their medical history, their family history, risks environmental conditions, and predisposition to degenerative diseases. In all the above parameters such as to time and dose to be applied are crucial be able to determine the therapeutic effectiveness. This is determined according to the type of trial or clinical expertise used to develop the application protocols focusing on the type of pathology. As of 2017 we had carried out 1,292 new observational tests in which the following criteria were evaluated:
Revitalisierung der Organe
CELLORGANE 4G hat Bio-Peptide in seine verschiedenen Präsentationen und Formeln integriert, um die Wirksamkeit dieser Behandlungen weiter zu stärken. Diese neuen Bestandteile erhöhen ihre therapeutische Wirksamkeit im Verhalten des Zellzyklus, da sie die Selbstregeneration von Geweben und Organen beeinflussen, Oxidations- und Reduktionsprozesse stimulieren und somit zu einem wahren Neutralisator für giftige Substanzen in den Zellen werden. Dies verleiht der Behandlung eine dreifache Aktivität in Bezug auf therapeutische Wirksamkeit, die zur Verbesserung der Funktionen von Organen und Systemen bei Dysfunktion oder Erkrankung aufgrund von Schwächung oder anderen altersbedingten Pathologien angezeigt ist.
Organische Alterungs- und Zellerneuerungstherapien
CELLORGANE 4G ist eine Begleitbehandlung zur Revitalisierung von Geweben und Organen. Tatsächlich handelt es sich um zelluläre Hyperernährung und/oder katalysierte zelluläre Ernährung, die die natürlichen Prozesse des Körpers zur Organregeneration stärkt und anregt und ein erschöpftes Organ revitalisiert.
Im Gegensatz zu den aktuellen konventionellen medizinischen Praktiken, bei denen synthetische Chemikalien verabreicht werden, ist die Therapie mit CELLORGANE 4G (die oft die konventionelle Therapie in vielen Krankheitsbildern ergänzt) wirklich eine biologisch natürliche Behandlung. Hier liegt zweifellos der "Kern" ihrer revitalisierenden Wirkung.
Die anderen Bestandteile ihrer Formeln aktivieren die organischen Reaktionen erheblich, indem sie nicht nur das geschwächte Organ direkt wiederherstellen und revitalisieren, sondern auch auf die übrigen Organe einwirken, die dieses Organsystem ergänzen. Sie haben mittel- und langfristige symptomatische Wirkungen, sodass ihre Anwendung im Rahmen langanhaltender Behandlungen bemerkenswerte Ergebnisse liefert.
Was ist Cellorgane 7?
Es handelt sich um eine biologische Behandlung in pharmazeutischer Qualität, die als Adjuvans in der Revitalisierung von Geweben und Organen wirkt, indem sie eine Zellhyperernährung und/oder katalysierte Zellernährung bietet. Sie stärkt und fördert die natürlichen Regenerationsprozesse, um das geschädigte Organ zu revitalisieren.
Welche Bestandteile hat Cellorgane 7?
Es handelt sich um eine Kombination aus opotherapeutischen Zellextrakten, Biopeptiden, Enzymen und Hormonvorläufern, die wichtige Reize in den Zellen erzeugen, um eine optimale Funktion verschiedener Organe und Systeme zu gewährleisten. Die Formel ist weltweit exklusiv und enthält keine chemischen Substanzen, Steroide oder synthetisch hergestellte Hormonersatzstoffe.
Wie funktioniert Cellorgane 7?
Es funktioniert nach dem Prinzip der "Wirkungsspezifität". Dies bedeutet, dass die Zellularextrakte bestimmter Organe in den Formeln, in Verbindung mit kurzen Aminosäureketten der Biopeptide, die Fähigkeit haben, das kranke Organ oder Gewebe des Patienten zu zielen (Organotropismus) und auf sie einzuwirken (Organospezifität), um eine Revitalisierung mit hohem Regenerationspotenzial auszulösen, insbesondere bei Personen mit Funktionsstörungen oder die von verschiedenen Faktoren wie Alter, Vererbung, Entzündungs- und Krankheitsprozessen betroffen sind.
Welche Nebenwirkungen und Reaktionen hat Cellorgane 7?
Es hat keine Nebenwirkungen, da seine Toxizität völlig null ist. Es verursacht keine unerwünschten Reaktionen und kann sicher in Kombination mit anderen Behandlungen eingenommen werden, da es nicht mit anderen Medikamenten interagiert.
Ist Cellorgane 7 4G in der Apotheke erhältlich?
Da es sich um ein Produkt biologischen Ursprungs handelt und nicht pharmazeutisch ist, ist es nicht in herkömmlichen Apotheken erhältlich, sondern nur in spezialisierten Apotheken.
Biopharmaxie und
Schweizer Klinik Biocell
Cellorgane 7
Indikationen:
- Migräne.
- Kopfschmerzen.
- Nervenschmerzen.
- Rückenschmerzen.
- Ischias.
- Endokrine Dysfunktion.
- Depression.
- Chronischer Stress.
- Zuckerkrankheit (Diabetes).
- Konzentrations- und Gedächtnisverlust.
- Parkinson.
- Alzheimer.
- Sarkopenie.
- Verbessert die kognitive Funktion und geistige Fähigkeiten.
- Verbessert die Kommunikation zwischen dem Gehirn und den Organen und Systemen.
- Verbessert die neurologische Reaktion.
Zusammensetzung:
Packungen mit 30 Tabletten à 650 mg / Packungen mit 60 Tabletten à 650 mg.
Jede Tablette à 650 mg enthält:
Opotherapeutische Zellextrakte: Gehirn, Hypothalamus, Hypophysendrüse (Hirnanhangdrüse).
Peptidextrakte von: Gehirn, Hypothalamus, Hypophysendrüse (Hirnanhangdrüse).
Enzymkomplexe: Superoxiddismutase, Glutathionperoxidase, Glutathionreduktase, Glutathiontransferase, Adenosintriphosphat (ATP).
Andere Inhaltsstoffe: Stabilisatoren und Hilfsstoffe.
Wirkmechanismus der 4. Generation von Zellen:
Der Wirkmechanismus von Peptiden ist ähnlich dem von Medikamenten, da die letzten drei Aminosäurereste neben dem C-Terminus der Peptide mit therapeutischer Aktivität stark an der aktiven Stelle binden, wo sie eine höhere Hemmungsspezifität aufweisen. Die Biopeptide sind in enterisch beschichteten Tabletten und Kapseln geschützt, um ihren Abbau im Magen-Darm-Trakt zu verhindern und die Absorption in den Darmwänden zu fördern, was die Absorptionsrate im Blutkreislauf verbessert. Dazu haben wir in jede Formel Katalysatoren und drei Aminosäuren, Arginin, Glycin und Asparaginsäure, eingebaut, um die chemische Reaktion des Körpers für die Kopplung und spätere Absorption zu steigern. Durch das Einbringen dieser Sequenz in verschiedene Peptidketten an verschiedenen Positionen des Formeltyps ermöglichen wir neue Varianten sowohl in der negativ geladenen Seitenkette der Asparaginsäure als auch in der positiv geladenen Argininseite. Diese Formel und Methoden entsprechen einem exklusiven Patent der Produkte der 4. Generation von Biocell Ultravital.
Pharmakokinetik und Pharmakodynamik:
Die Bestandteile der verschiedenen Formeln erreichen die Zellen entweder direkt über das Blut oder indirekt bei oralen Produkten und werden von ihnen aufgenommen, je nach Größe der Moleküle der jeweiligen Elemente, durch verschiedene Zelltransportmittel. Bei größeren Molekülen erfolgt die Aufnahme in die Zellen durch rezeptorvermittelte Endozytose, wobei der Ablauf ist: Vesikel, Endosom und Lysosom. Bei kleineren Molekülen erfolgt die Aufnahme durch einfache Diffusion oder erleichterte Diffusion durch Proteine, je nach Fall.
Die Zellextrakte, die durch Endozytose in die Zellen gelangen, bestehen aus Molekülen, bei denen die Atome durch chemische Bindungen verbunden sind, bei denen die Energie gespeichert ist. Sowohl die Materie als auch die Energie werden von der Zelle durch einen Prozess namens zelluläre Verdauung verwendet, bei dem die Moleküle durch die Wirkung von Hydrolasen, die in den Lysosomen enthalten sind, abgebaut werden.
Verabreichung:
Die orale Anwendung erfolgt vor den Mahlzeiten zur Verbesserung der Absorption, vorzugsweise mit Wasser.
Dosierung:
Zur Prävention bei gesunden Menschen wird empfohlen, täglich sechs Monate lang morgens und abends je eine (1) Tablette einzunehmen, abwechselnd oder aufeinanderfolgend. Bei Personen mit einer der hier aufgeführten Erkrankungen oder Beschwerden wird empfohlen, zusätzlich zu den vom behandelnden Arzt verschriebenen Medikamenten täglich zwölf Monate lang morgens und abends jeweils zwei (2) Tabletten einzunehmen. Es kann unbegrenzt eingenommen werden, da seine Bestandteile keine Ablagerungen bilden.
Nebenwirkungen:
Aufgrund des Peptid- und Opotherapiegehalts in der Formel kann es in einigen Fällen zu leichten Kopfschmerzen kommen, die nach wenigen Minuten verschwinden; Übelkeit tritt sehr selten auf und verschwindet nach wenigen Stunden.
Extraktion und Gewinnung von Biopeptiden und Zellextrakten:
Es ist wichtig zu beachten, dass die Formeln biologische Bestandteile tierischen Ursprungs in Kombination mit anderen Verbindungen enthalten, die die therapeutische Wirkung verbessern und speziell in embryonalem Stadium extrahiert und im zweiten Monat der Schwangerschaft verarbeitet werden. Diese Bestandteile, die in einigen unserer Produkteformeln enthalten sind, werden nach einem langen biochemischen Prozess gewonnen und sind zertifiziert frei von Prionen, Pyrogenen, Bakterien, Nanobakterien, Pilzen, Viren und nach zahlreichen Kontrollen sind jegliche Risiken einer immunologischen Reaktion beim Menschen zu 100% beseitigt. Im Laufe der Jahre hat sich gezeigt, dass Schweine und Schafe die besten Spender sind, da sie stark und immunologisch widerstandsfähig sind. Dies wird durch die Tatsache belegt, dass die meisten Herzklappentransplantationen für Menschen selbst im 21. Jahrhundert von Schweinen stammen, zusätzlich zu Insulin und vielen anderen therapeutisch verwendeten Produkten, die von diesen Tieren stammen. Diese Methoden werden kontinuierlich überwacht, um sicherzustellen, dass sie den strengsten Anforderungen an die Biosicherheit für therapeutische Zwecke entsprechen. Alle Bestandteile und anderen aktiven Inhaltsstoffe der Formeln sind ebenfalls von der
US-amerikanischen Lebens- und Arzneimittelbehörde (FDA) zugelassen und werden in den USA von
BIOCELL ULTRAVITAL USA unter schweizerischer Lizenz von
Biocell Ultravital hergestellt.
Gegenanzeigen:
Es kann sicher mit anderen Medikamenten kombiniert werden. In den empfohlenen Dosen wurden in keinem Fall unerwünschte Reaktionen beobachtet. Personen mit Diabetes sollten vorher überwacht werden.
Präsentation und Verpackung:
Enthält eine Schachtel mit zwei (2) Blisterpackungen oder vier (4) Blisterpackungen mit jeweils 15 Tabletten à 650 mg, und die Herstellung erfolgt in einer sterilen Umgebung gemäß den internationalen Vorschriften.
Lagerung:
An einem trockenen und kühlen Ort aufbewahren, bei Raumtemperatur zwischen 5°C und 40°C. Jede Tablette hat eine Haltbarkeit von fünf (5) Jahren ab Herstellungsdatum.
Endproduktkontrolle:
Zwei (2) unabhängige Labors führen verschiedene Tests und Kontrollen auf Pilze, bakteriologische Analysen des Typs 5, multizentrische immunzelluläre Tests, Brucellose-Tests, Virentests, zahlreiche Prionentests, Bakteriosen, Cyclosporen, Mykobakterien, Salmonellen gemäß biologischem Material für therapeutische und menschliche Verwendung gemäß den aktuellen Vorschriften der EU durch.
Warnung:
Biocell Ultravital garantiert die Reinheit und Qualität seiner Produkte und ist nicht verantwortlich für Schäden, die durch fahrlässige Praktiken Dritter verursacht werden.
How Does it Work?
How the Formulas are Developed?
All our products have been formulated to optimize cell function because if we have healthy cells, we will be healthier every day, and they are based on our 4 pillars of cellular longevity. They work together as a whole to promote energy production and cell growth.
We strive to achieve perfection for each of our therapeutic categories, since they are formulated by carefully selecting each component after confirming its characteristics and clinical trials that positively compromise the efficacy in each formula, this is basically the science on which our products and therapies are based.
Biocell Ultravital products are formulated with ingredients that are 100% natural biological substances, with the highest quality standards, containing cellular extracts of animal origin and peptide hydrolysates extracted from plant extracts and glands of young animals in combination with antioxidants, enzymes and vitamins we managed to optimize the formulas and their therapeutic scope.
All raw material sources are carefully selected from organic plants and organs of pigs and sheep less than one year old and undergo a prion and decontaminated pharmaceutical standardization process that meets high biosecurity standards for reach specific concentrations of high purity and are processed under strict controls to guarantee the high quality and purity of their products.
No chemicals, other harmful or unwanted substances are used in the extraction. Modern technology ensures the recovery of assets in a biologically natural way, without changes. This is very important for the safety of each formula. None of the components in Biocell Ultravital formulas are genetically modified. (NON GMO)
Production and Extraction of Bio Peptides 4th Cellular Generation
The chemical synthesis to produce bioactive peptides of the 4th cell generation of
Biocell Ultravital are obtained using combined methods of enzymatic hydrolysis through proteolytic enzymes and by fermentation using starter cultures. This combination of both methods gives origin to peptides with a safer biological activity due to the fact that in one all the contaminating traces are eliminated and the other facilitates the absorption through the intestinal tract. An additional advantage of enzymatic hydrolysis is the reduction of allergens. The procedure to obtain, isolate and identify biopeptides with specific biological activity, is obtained with the solution of the gland or organ to extract biopeptides, to which papain, pepsin, trypsin are added, the application of different enzymes pursue the formation of a mixture with different ranges of biological activity and incubated for a certain time to obtain the desired degree of hydrolysis.
After this, the hydrolyzate is fractionated by exclusion chromatography and semi-preparative liquid chromatography in reverse phase, selecting the fraction with the highest activity as a result of the first invitro tests, and finally the sequence of the peptides responsible for generating the activity is identified. The desired therapy is obtained by applying mass spectrometry and / or N-terminal sequencing. However, hydrolysates are complex mixtures and can contain up to hundreds of different molecules, therefore, locating bioactive peptides in these procedures is a biochemical work of constant expertise that results in a difficult task and requires a lot of time and dedication. The fractions usually still contain multiple compounds that require additional cycles of fractionation, concentration and evaluation of bioactivity to be able to identify the molecule responsible for the activity in the formulas of each product according to the therapeutic indication.
Consequently, the enzymatic hydrolysis used offers undoubted advantages, such as the absence of substrate degradation processes since the enzymes are selective for a type of bond, pH values between 5 to 10 and the temperatures between 40 to 60ºC, thus maintaining or improving the nutritional value of the protein. The specificity of the enzyme affects the size, quantity, and composition of free peptides and amino acids, as well as their amino acid sequence.
Safety in Therapies
Safety control is a very important factor, especially when it comes to high quality health products, which is why at
Biocell Ultravital in France, which is the main headquarters where all the research and development of the formulas take place, we have monitoring and follow-up mechanisms for each product, this to prevent any error in the next production, that is why we are continuously implementing software for the detection of technical failures of the human team in charge of production. This translates into maximum safety controls that guarantee the complete quality of the production, eliminating 100% possible adverse reactions due to the indication of some of our products that does not correspond to a contraindication already described.
This discipline has become responsible for quality control in the formulation and manufacturing phases thanks to the detection, evaluation, knowledge and analysis for the prevention of adverse reactions and other possible problems related to the products, covering a wide spectrum of continuous analysis so that they do not cause any harm to the patient. We actively participate in decision-making when manufacturing to prevent possible complications in any of the production stages. Thanks to the standardization and development of specific protocols established for each production and subjected to continuous analysis adhering to very specific guidelines we are able to prevent these unwanted complications. We are mandated by compliance with the European Pharmacopoeia in Europe and adhere to current FDA regulations when manufacturing the different products corresponding to Biocell Ultravital Cellular Renovation Therapies.
Research and Development
When
Biocell Ultravital incorporates products with new formulas and they reach the market, at least an average of 5 to 7 years have elapsed, consumed in the different phases of research and development. This journey requires a scientific and economic effort, for this reason we say that out of every 300 new formulas that are beginning to be investigated, only 20 end up becoming treatments available to patients either as an adjunct or preventive of cellular function and the immune system.
Working in the context of formulas based on opotherapeutic extracts or cells of xenogeneic origin, they aim to support the expansion of products for basically strengthening the immune system.
Cellular Renovation Therapy may contribute as reinforcement to the primary treatment to treat the various human diseases that are associated with failures of the immune system. In order to achieve this purpose the continuous efforts of a scientific committee structured by various institutions in the field biology headed by Prof. Dr. Ben L Pfizer is needed. These contributions guide new formulations that improve the components has been allocated to four fundamental guidelines with specific objectives corresponding to the 4th generation cellular products by Biocell Ultravital: Protection, Repair, Revitalization and Regeneration properties. These therapeutic lines are developed with a common research and development strategic plan for each product category.
Manufacturing Plants and Procedures
In Europe, research, production and development is carried out at Biocell Ultravital, France, and we share part of the manufacturing in two other associated plants, mainly for the production of some raw materials, between Germany and the Netherlands.
We also manufacture our products in the USA, BIOCELL ULTRAVITAL USA Laboratories, where we have a modern production factory especially for manufacturing formulas and products for oral presentation.
Since our products formulas require components be extracted from a biological source, in all manufacturing plants we have ensured that they are manufactured in production rooms that have state-of-the-art equipment controlled by software that manages the procedures monitoring each phase of the process. Development with the latest air conditioning, quality and safety systems, to ensure that the final product does not lose its therapeutic potency, for this reason the active ingredients of each formula and the preparation in the initial phase are conditioned in isolated clean rooms and by specialized professionals trained to measure and control the production process from the moment this first manufacturing phase begins and continuing these quality controls through each phase of production until the final product is approved.
Each manufacturing plant, although with different facilities, produces with the same manufacturing procedures, efficiently following the highest quality standards that comply with all the regulations established by the European Pharmacopoeia and the FDA.
Products that Improve Cellular Renovation
Currently there is a growing interest in cell therapy extracted from the patient’s own stem cells, unlike our products,
Cellular renovation therapies (CRT) provide sustained results without complications and represent a great economic saving in comparison. The products in the Biocell Ultravital line of
Cell Renewal Therapies are divided into 5 main groups that include:
Regeneration, Revitalization, Detoxification, De-inflammatory and Hormonal.
CRT have worked with great success in recent years as a preventive treatment, without discarding its effective action as a primary therapeutic. The treatment benefits are notorious for their adjuvant action in allopathic medicine. CRT, through their formulas, exclusively allow us to transform diseased or aging organs into healthy organs with a highly functional capacity. Their components activate a series of stimuli that cells need for a normal cycle of continuous renewal, and they can be incorporated into the body through injections or orally. These various components are assimilated by the body through endocytosis and a good part is introduced into the cells. This is produced by the emission of pseudopods until they completely encompass it to form a vacuole, which then fuses with the lysosomes to degrade the phagocytosed substance, which is known by the name of phagosome. This method is characterized by being the mode of nutrition used by cells through ingestion of foreign matter. In addition, it is one of the greater means of transport that they use to defend themselves against some cells of multicellular organisms.
From this mechanism and many other stimulating biochemical changes, nutrition begins for an effective CELLULAR RENOVATION. CRT brings a fresh genetic information contained in DNA and RNA, stimulating secretion inducing old or sick cells and reprogramming to operate properly, providing the recipient bodies, a large number of biochemical and enzyme substrates containing information needed to revitalize an organ, or a gland. CRT contains various compounds that are primarily responsible for properly nourishing cells, but, moreover, each of the formulas in a CRT contains cells and embryonal tissues that increase positive changes in cell cycle complementing in part dysfunctional lapses. This is how the different components are first incorporated as essential nutrients and as well, how the embryonic tissues reach the organ with low vitality and that need to be dynamized. This process is called CELLULAR RENOVATION and its benefits are perceived after several weeks, as it passes slowly from the cell to the tissue, from the tissue to the organ and from the organ to the system. Some specialists feel that the most important result of using CRT is the revitalization of the body’s immune defense mechanisms. When damage occurs to the cells that make up the different tissues and organs involved in the immune system, either through aging or environmental poisoning, the body becomes defenseless from both external invasion and internal degeneration. The damage caused to the organs of the immune system can be reversed by stimulating the body’s defense mechanisms to boost the health of the weakened organ.
Security System to Prevent Falsification
Biocell Ultravital has developed for the
4th generation a sophisticated security system for its products to prevent the repetition of copies and / or counterfeiting of our products. Moving forward, we will invest a great technological effort in each package to ensure that you will have an original product in your hand.
This security system is applied to new packaging that have a QR Scan code applied to a double security system, a first physical stage in the packaging that will reveal through a security tab if the product has been violated and a second with an implantation of sophisticated software that will allow you to check if the product you purchased is original through our website, or by downloading the new BIOCELL APP for Android and IOS for free.
The falsification of ethical and health products is a problem that is becoming increasingly important, and all the different therapeutic areas may be affected.
These counterfeits seek to pass for authentic products, copying the packaging material, the commercial image, the logo, the information brochures, as well as the shape and colors of the original product.
In addition, they use the same lot number, expiration date and other identifying elements of the authentic product. However, they are manufactured and placed on the market without the authorization of any health authority. These original and generic medicines, as well as vitamins are also falsified and even more so those products that enjoy prestige are the most vulnerable and these falsifications may contain active ingredients in incorrect doses or lower doses than those authorized, or the absence of active ingredients and even include some toxic elements. Many may even be manufactured under dangerous conditions that are not controlled. Thus, they can pose a significant risk to health and are sometimes especially difficult to identify.
In short, they look identical to the original product, but contain “uncontrolled” components that are life threatening.
he trade in counterfeit medicines is controlled by international criminal networks, and no region is exempt. Those countries with less controlled drug distribution systems are more vulnerable to this type of practice; However, the most developed countries are not spared from this evil, especially when patients purchase drugs on the internet or unauthorized websites. Unfortunately, in the past we have been victims of these unethical organizations and have had to report these facts to the authorities since many people especially in Asian countries, as well as Mexico and others in Central and South America are victims of misleading offers of therapies, even by unethical doctors who offer the products at prices lower than the real cost. However, in the end they spend more money due to the need to seek immediate clinical help as demonstrated on several occasions reported by us through our attorneys around the world from patients who were treated clinically for administering fake products.
















